It’s a truism that blue skies research, pursuing questions merely because they’re interesting, often leads to major advances in medical knowledge; many of the stories in Blue Skies and Bench Space attest to this, and they’re just a small sample of the accrued scientific wisdom on the subject.
There’s an obvious reason for the strong relationship between basic research and illness: the diseased state, where cells and bodies are thrown out of kilter and start to behave strangely, is the perfect place to find important molecules and processes – the mechanisms which regulate normality are more easily revealed when they aren’t working properly. Conversely, if one is working in a normal system, or in an organism far-removed from ourselves, importance can be demonstrated by finding a disease in which your molecule of choice is implicated.
Being able to wrap their obsessions in the altruistic cloak of medical relevance is extremely convenient for the scientific community, as curiosity-driven research is exciting and intellectually stimulating. Cancer researchers have been especially lucky in this regard. In the early days, as cancer cells are extremely easy to grow in bulk culture in the lab, they were the perfect tools for exploring the basic mechanisms of cell growth, thereby delivering the twin goals of fascination and medical relevance. These days, the more we learn about the molecular basis of cancer, the more interesting it gets. It’s both important and satisfying designing fancy model systems to explore initiation, progression, growth, and metastasis, thinking how to target drugs to specifically disable key molecules, and tying it all in to the mass of data being generated by our ability to quickly analyse pretty much every aspect of the biochemistry and genetics of a tumour. To have that much fun, with the added bonus of helping to prolong life, is a huge privilege, which in general is truly appreciated by the cancer research community.
Whilst blue skies research is going to be worth funding for a long time to come, for all the reasons stated above, a few clouds are massing on the cancer horizon. As we find out more and more about the biology of advanced cancer, it’s becoming clear that there will never be an easy fix. There will be no magic bullets, and even if there were, cancers are scarily adept at dodging therapies, however targeted and sophisticated, springing up anew by evolving their way out of tight corners. There is an additional problem: targeted therapies are extremely expensive, and we are bracing ourselves for an upsurge in cancer in the developing world. The financial resources to treat everyone with the most effective therapies simply do not exist.
So how should we proceed? There is increasing agreement that the way to combat cancer lies in a shift in focus: rather than concentrating so heavily on the endgame, we need to do more research into prevention and early diagnosis. More than thirty years ago, Richard Doll and Richard Peto suggested in a landmark report that around 75% of cancers might be preventable, and this likely still holds true. Similarly, it’s estimated that a further 15% of cancers would be treatable if caught at an early stage. If only 10% of cancers got to the advanced stage, the cancer burden on the exchequer would be greatly reduced, making it possible to deploy the full arsenal of anti-cancer therapies.
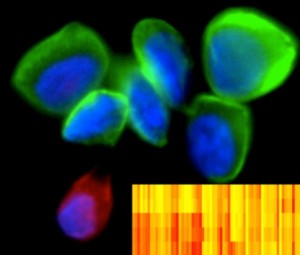
DNA barcoding of cancer. Image credit: Ralph Weissleder, Center for Systems Biology, Massachusetts General Hospital, Boston
Research into early diagnosis of cancer has never been a particular priority of the ferociously ambitious basic scientist, as it was regarded as boring, thankless drudgery. Looking for biomarkers able to flag early, premalignant changes in cells was snobbily regarded as the sort of thing that mediocre applied research labs did to earn a crust. However, in today’s multidisciplinary world, the distinction between basic and applied science is rapidly disappearing, and screening and early detection is now a fertile field in which biophysicists and biologists are combining to develop new imaging modalities, and cheaper, less invasive replacements for the traditional biopsy.
There’s a lot going at present, and I’m only going to cite two examples that in my biased opinion, can’t fail to enthuse even the most hardened basic scientist – deploying cool techniques in an smart way to detect disease is just plain exciting.
First off, there’s a new method, developed at the MGH Center for Systems Biology in Boston, for rapidly identifying hundreds of cancer-related protein markers from tiny numbers of cells, using antibodies linked to unique DNA “barcodes”. The technique is both powerful and incredibly sensitive, and could be invaluable for helping doctors make diagnoses, follow disease progression, and track the efficacy of therapies, with only minor discomfort to patients, as minimally invasive methods can be used to remove the small numbers of cells required.
On my side of the pond, Molly Stevens at Imperial College London has published a method that may make early diagnosis accessible, accurate and cheap enough to be used in poorer countries. She and her lab have used nanotechnology to reinvent the standard Enzyme-Linked Immunosorbent Assay (ELISA). In a normal ELISA, antibodies are used to bind molecules of interest, triggering a chemical reaction that is detected by an expensive machine. Stevens and her colleagues have worked out how to eliminate the machine reader, by making the colour change visible to the naked eye. The new plasmonic ELISA is incredibly sensitive too – a millionth of a trillionth of a gram of protein per ml of human serum can be detected. If that isn’t nanotastic, I don’t know what is.
Prevention research is still lagging behind the newly supercharged early diagnosis field. Much of it is still the domain of epidemiologists and behavioural psychologists, who seek to define causes, and then persuade people to lay off their bad habits (although the development of medical avatars, virtual clones able to demonstrate what your current behaviour will do to your future health, is beginning to interest the biocomputing and wet biology communities). Research into possible anti-cancer dietary supplements, or the efficacy of taking drugs such as aspirin, is ongoing, although to be honest, it’s not regarded as a sexy subject – the methodology and length of time it takes to gather meaningful data make for extremely delayed gratification. Furthermore, sharing a research field with the wide selection of charlatans and chancers who peddle useless and expensive quackery is extremely offputting to many who could redress the balance in favour of proper science. This is clearly a problem, which does not yet have a good solution.
So what in prevention research might interest a biologist with a selfish desire to get speedy results, do some cool stuff and simultaneously save lives? The development of vaccines is one avenue. One in five cancers in the developing world has an infectious origin, and vaccines, such as those against cervical (human papillomavirus) and liver (hepatitis B) cancers are already in use to marked effect (although further education about the HPV vaccine is still necessary to combat the toxic cocktail of pardonable ignorance, questionable moral objections, and daft anti-scientific superstition that is preventing maximum uptake). Further vaccines against hepatitis C for liver cancer, and helicobacter pylori for stomach cancer, are also in the pipeline. One of the greatest challenges is to develop a vaccine for Epstein Barr Virus. EBV, first discovered 50 years ago this month by Antony Epstein, Yvonne Barr and Bert Achong, and worked on extensively by LRI stars Tomas Lindahl and Beverly Griffin, causes an estimated 200,000 new cancers worldwide every year; eliminating its threat would be a wonderful achievement.
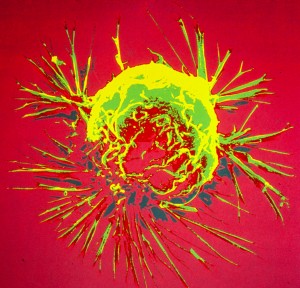
Breast cancer cell photographed by scanning electron microscopy. Source: US National Cancer Institute
What else? Although it is controversial, some researchers believe that unravelling the mysteries of breast cancer will one day allow the development of a hormonal treatment to protect women from cancerous changes; the prospect of averting almost a quarter of women’s cancers is an alluring one, however sceptical one might be. Extrapolating from this, one reaches an even more exciting (and far-fetched) possibility; perhaps our present forensically detailed investigations of the biology of cancers and precancerous changes may lead us to targeted methods of prevention, just as they have given us targeted therapies for later-stage cancers. Are we on the verge of the next big revolution – having the molecular knowledge to stop cancer before it even begins?


Discussion
No comments yet.